London Dispersion Forces Water
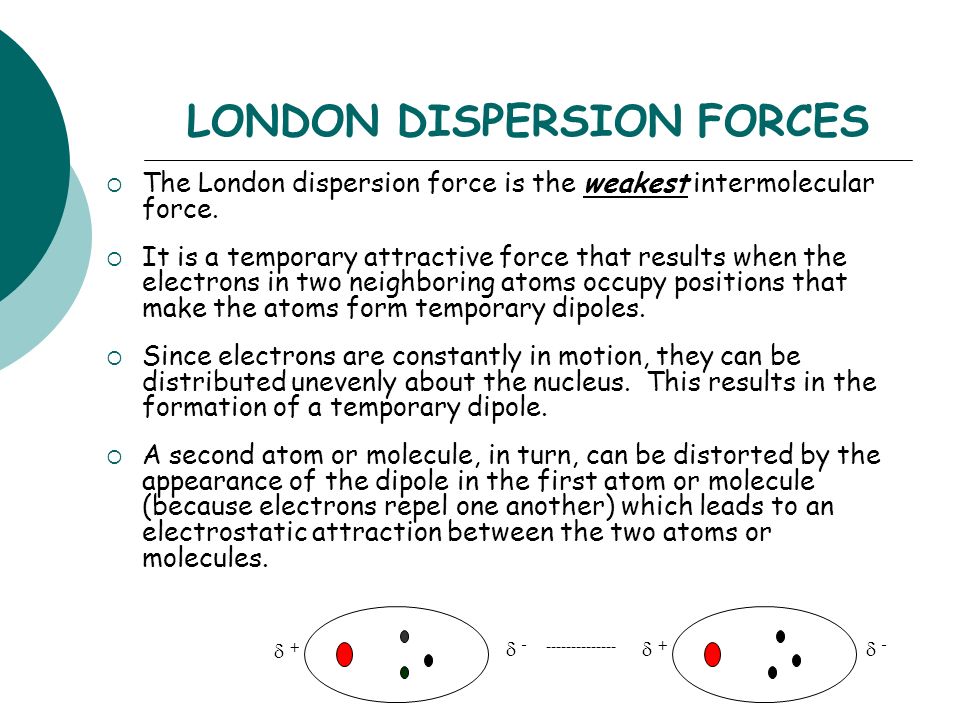
The london dispersion force is the weakest intermolecular force.
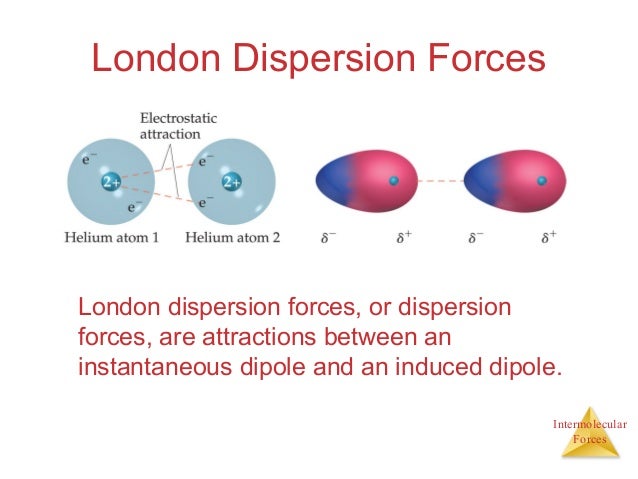
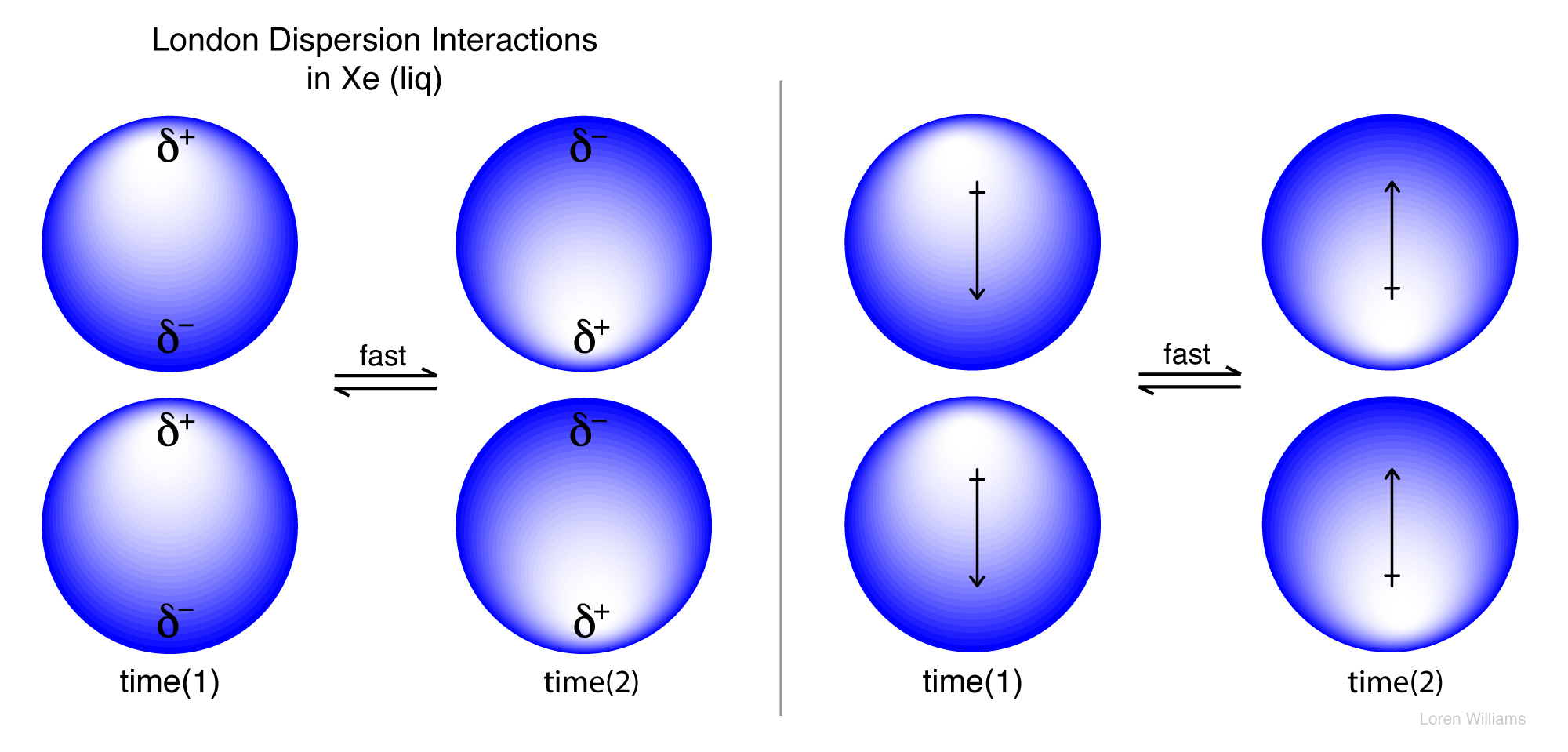
London dispersion forces water. The ldf is named after the german american physicist fritz london. This force is sometimes called an induced dipole induced dipole attraction. All things have london dispersion forces the weakest interactions being temporary dipoles that form by shifting of electrons within a molecule. The london dispersion force is the weakest intermolecular force.
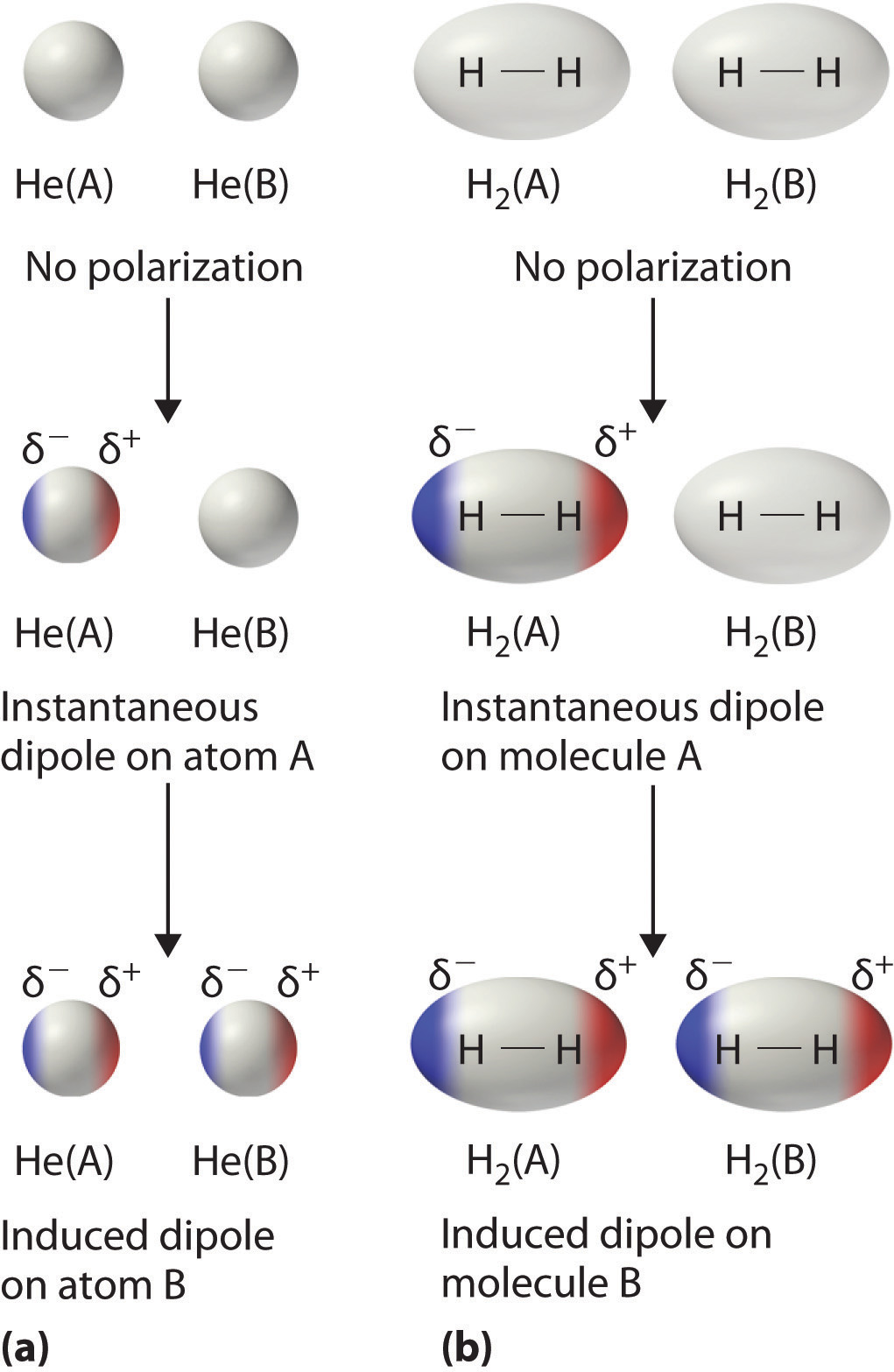
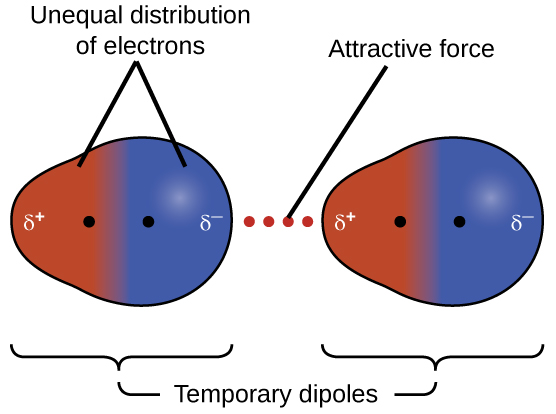
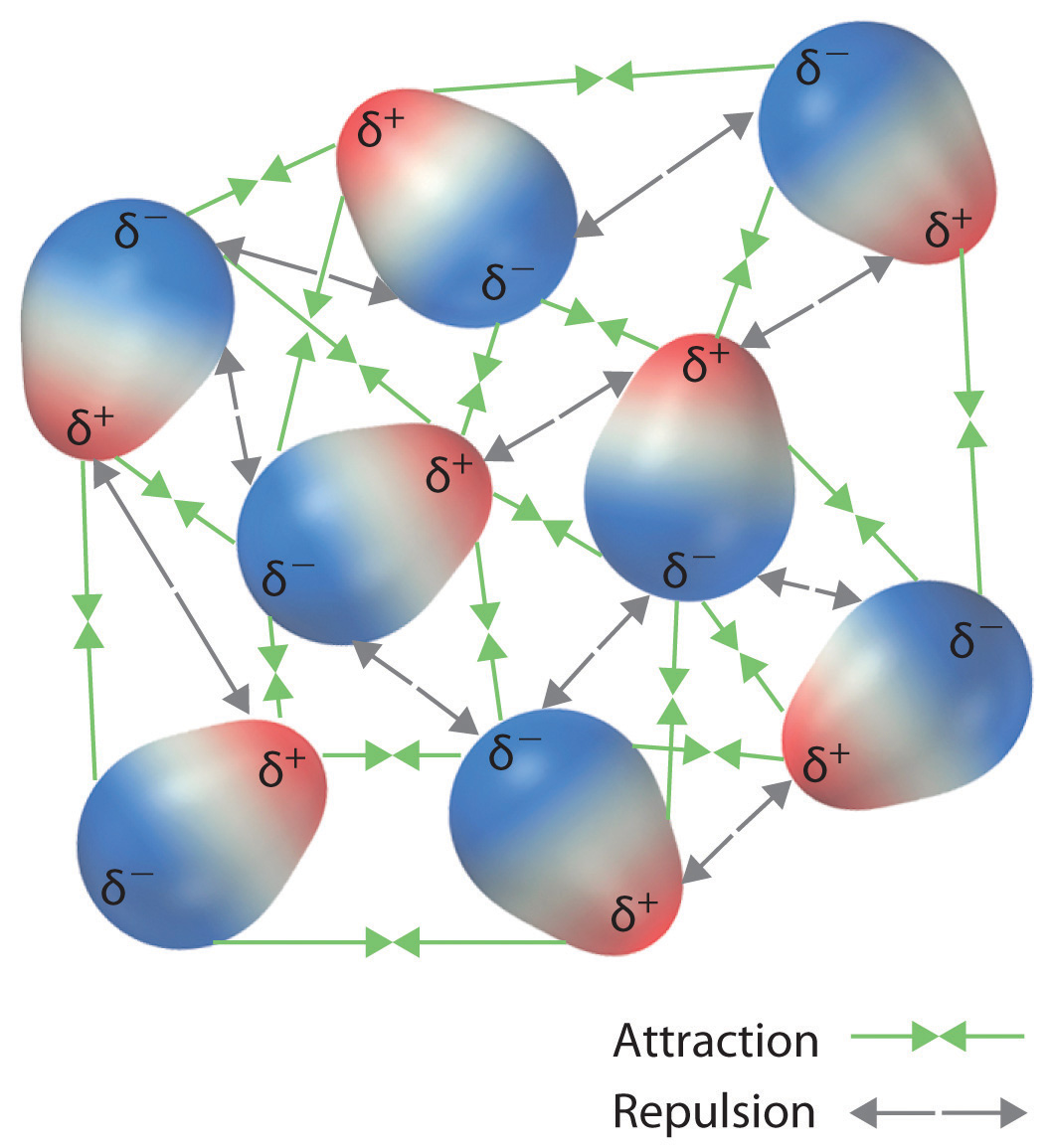
The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently. These interactions are generally called dispersion forces. London dispersion forces ldf also known as dispersion forces london forces instantaneous dipole induced dipole forces or loosely van der waals forces are a type of force acting between atoms and molecules.
They are one of three van der waals forces but are the only force present in materials that don t have polar dipole molecules. They are part of the van der waals forces. The more electrons a molecule has the stronger the london dispersion forces are. There is a very small difference in the electronegativity between carbon and hydrogen that can be disregarded.
It is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.